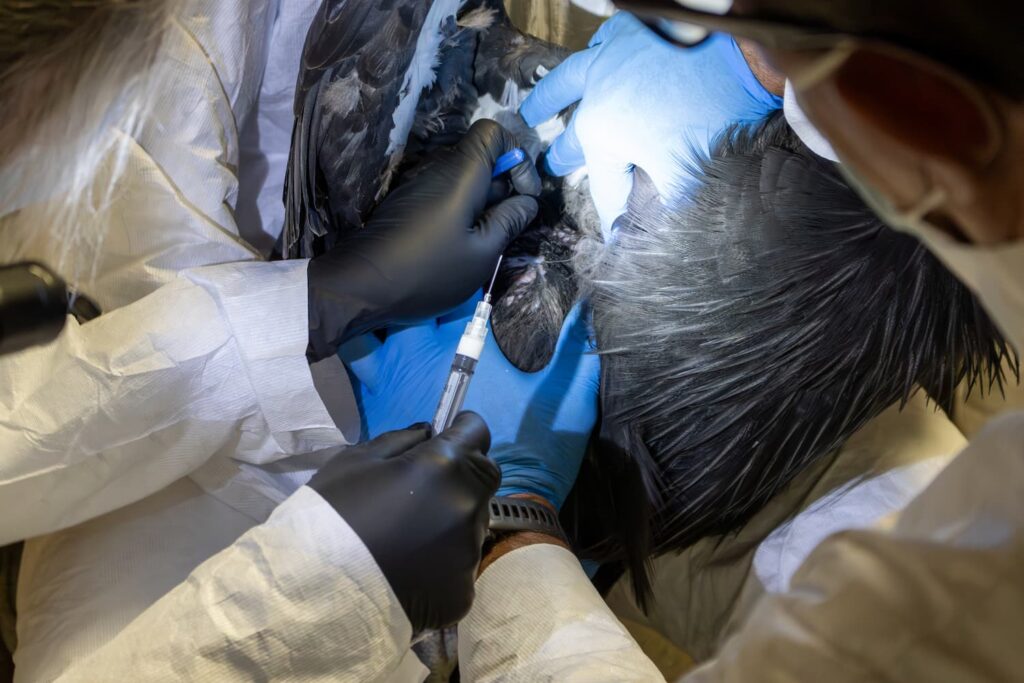
Birds from the L.A. Zoo, San Diego Zoo Wildlife Alliance, and the Oregon Zoo participated in historic trials, furthering condor recovery efforts
The U.S. Fish & Wildlife Service (Service) is sharing early results from the historic vaccine trial for highly pathogenic avian influenza (HPAI) in California condors (Gymnogyps californianus). As reported by the Service’s Incident Command leading the effort, results from the first test group showed that 60 percent of the condors produced measurable antibodies that are expected to provide partial protection against mortality from HPAI if the birds are exposed.
The trial, developed in coordination with the Service, U.S. Department of Agriculture (USDA) and U.S. Geological Service was carried out by the Los Angeles Zoo (L.A. Zoo), San Diego Zoo Wildlife Alliance (SDZWA), and the Oregon Zoo as longtime California Condor Recovery Program partners, and with a new recovery partner, the Carolina Raptor Center.
HPAI was detected in condors in Arizona in early April 2023, although it has been observed in many other species throughout the United States. There were 21 condor mortalities during this HPAI outbreak. In May, the USDA’s Animal and Plant Health Inspection Service approved the emergency use of a vaccine against HPAI to be piloted among the critically endangered California condors in managed care. These first results from the L.A. Zoo and SDZWA show that the vaccine may have beneficial effects for free flying California condors. The Service will make a determination on vaccinating free flying condors after the final results from the trials are available.
“We are grateful to be working with such high caliber professionals as we have been evaluating this vaccine for potential use to minimize impacts from HPAI on the condor recovery efforts. Collaboration with our zoological partners has been vital for the implementation of this trial and their ongoing support is essential for implementing the first vaccines in pre-release condors this fall and winter. None of this important work would be possible without the collaboration from all our partners,” said Ashleigh Blackford, California Condor Recovery Program Coordinator for the Service.
Each zoo has worked closely with the Service’s veterinarian, USDA, and state veterinarians to gain approval to participate in this first trial for HPAI vaccines in the United States for wild birds. The trial evaluates two vaccination approaches – a single dose (1 ml) and an initial dose with a booster shot (0.5ml on two occasions). The vaccine used in this trial was developed and manufactured by the animal health company Zoetis.
Twenty-five condors spread across the three zoos were identified for participation based on the birds age, gender, genetics, and other factors (e.g., some condors are still raising young of the year). Of those 25, 10 condors received two doses of the vaccine, 10 condors received a single vaccination and five condors served as the control group. The early results shared and the Service’s decision to vaccinate pre-release birds this year are based on the group that received 2 doses of the vaccine. Presence and degree of immune response to the vaccine were evaluated using a hemagglutination inhibition assay, an antibody detection test, performed at USDA’s Southeast Poultry Research Laboratory.
“Just over 30 years ago, the L.A. Zoo joined the U.S. Fish and Wildlife Service and its partners to help save and protect these critically endangered birds. The species was on the brink of extinction and our work was vital to the recovery – increasing the population from approximately 20 in the 1980s to more than 500 California condors in the world today,” said Denise M. Verret, CEO & Zoo Director, Los Angeles Zoo. “This vaccination trial is an urgent next step in the partnership to protect these birds from a new, existential threat – the highly pathogenic avian influenza virus. It gives these resilient birds a fighting chance and provides hope for a future where the species is once again thriving in nature.”
“When our California condor conservation breeding program began in the late 1980s, we embarked on a dedicated journey to save these incredible birds from existential threats and to offer them a sustainable future,” said Hendrik Nollens, Vice President of Wildlife Health, San Diego Zoo Wildlife Alliance. “We see this as another chapter in that journey, and as one of only a few organizations who care for California condors, we are uniquely positioned to help.”
The vaccine trial first started with a surrogate species, black vultures, hosted at the Carolina Raptor Center, North Carolina. This step in the trial signaled that the vaccine was safe in vulture species and with expert input, trials could proceed in condors
After, confirming vaccine safety with the surrogate species, on July 18, the Service, L.A. Zoo veterinarians, and zoo team vaccinated the first three condors, to evaluate their response. After further confirming there was no adverse reaction in condors, the L.A. Zoo, SDZWA, and Oregon Zoo vaccinated 17 additional birds. More information about the overall trial, next steps and impacts of HPAI on the Southwest flock can be found on the Service’s web page dedicated to HPAI updates. The Service is expected to announce final trial results and additional updates on implementation on this platform.
“The importance of finding a vaccine that’s effective in protecting California condors cannot be overstated,” said Dr. Carlos Sanchez, Director of Animal Health, Oregon Zoo. “This is a species that not long ago was on the very brink of extinction. Just over 300 individuals exist in the wild — and this year, in a matter of weeks, HPAI wiped out 21 of them. If left unchecked, the disease could undo decades of conservation work in the blink of an eye.”
The California condor recovery program has long been an example of how partnerships can help promote recovery of endangered species. The effort to protect condors against HPAI highlights the value of partnerships once again incorporating new and novel partners and creative ideas to work towards successful recovery.
“We’re thrilled to have been able to play a role in this process and are excited to see the California condor trial is proving to be successful,” says Erin Katzner, President and CEO, Carolina Raptor Center. “We hope the black vultures can continue to provide information to aide in the decision-making process as the team determines next steps.”